A Luminous New Dawn for Parkinson’s
Parkinson’s disease doesn’t just affect the body — it slowly reshapes a person’s life. From the first tremor to difficulty walking, speaking, or even smiling, this progressive neurological condition changes how a person experiences the world.
Parkinson’s and the Quiet Rise of Regenerative Hope
In the soft glow of scientific progress, the story of Parkinson’s is being reshaped—no longer defined solely by pills, patience, and progressive decline, but illuminated now by the quiet light of regenerative stem-cell therapy.
For decades, Parkinson’s was managed but not mended; calmed but not reversed. Now, in advanced laboratories, within sterile vials and delicate cultures, something extraordinary is unfolding: the possibility of neuronal healing, not just suppression.
These therapies do not shout—they whisper from microscopes, from early-stage clinical trials, from the slowed tremor of a real patient who once shook with fear but now holds a spoon with ease.
We share this not to raise expectations but to awaken awareness. Not to promise miracles, but to honor the milestones science is making—gently, humbly, and together with patients, not apart from them.
Because in this new chapter, hope is not a marketing line. It is a clinical protocol. It is data with a pulse. It is a hand reaching out—not to cure overnight—but to accompany you into the future with more understanding, more dignity, and a little more light.
What Is Parkinson’s and Why Stem‑Cell Therapy Resonates
Parkinson’s is a progressive neurodegenerative disorder rooted in the gradual loss of dopamine‑producing neurons in the substantia nigra of the brain. This leads to motor symptoms like tremor, rigidity, slowness, and postural instability, as well as non‑motor features such as sleep disturbance, emotional changes, and cognitive decline. Traditional therapies—particularly levodopa—help with symptoms but cannot halt neuronal loss or restore lost cells.
Stem‑cell therapy offers a hopeful alternative: the replacement or protection of those very dopamine neurons lost to Parkinson’s. This can take several forms:
- Induced pluripotent stem cells (iPSCs) derived from a patient’s own cells, reprogrammed into midbrain dopaminergic neurons. These offer immunological compatibility and avoid rejection or immunosuppression. As described in a Phase 1 U.S. trial run by McLean Hospital and Mass General Brigham, researchers reprogrammed patients’ own blood‑derived cells into iPSCs, then into dopamine neurons, and transplanted them into the brain to test safety and early signs of function (UC Irvine School of Medicine), (UCI Health tests novel stem cell therapy for advanced Parkinson’s disease), (Clinical Trial Tests Novel Stem-Cell Treatment for Parkinson’s Disease)
- Embryonic stem cell‑derived dopamine progenitors, tested in early trials such as exPDite at UCI and NeuroTherapeutics in Toronto and New York. Published April 2025 in Nature, this Phase 1 trial confirmed safety at 18 months and observed some patients with visible tremor reduction and symptom relief. (Stem-cell therapy is a ‘big leap’ for Parkinson’s treatment)
- STEM‑PD, a trial in Europe (Lund University, Cambridge) transplanting stem‑cell–derived dopamine neurons into eight Parkinson’s patients over 36 months, has recently advanced from Phase 1 into next stages based on promising early safety data (The STEM-PD trial has recently published an update, which indicates the team is proceeding to the next trial stage based on early positive safety data.)
- ANGE‑S003, intranasal transplantation of human neural stem cells in China: a Phase 1 study with 18 advanced Parkinson’s patients, four doses delivered via nasal route. The trial showed no serious adverse events and significant improvement in unified Parkinson’s rating scale (MDS‑UPDRS), peaking at six months and sustained through a year (Chinese studies on stem cells for Parkinson’s disease and sepsis)
- https://pmc.ncbi.nlm.nih.gov/articles/PMC7663462/Mesenchymal stem cells (MSCs)—from bone marrow, adipose, or umbilical cord—are under numerous early trials worldwide, with anti‑inflammatory, neuroprotective and trophic effects. These include trials in China, Jordan, India, USA, Mexico. (Stem Cell-Based Therapies for Parkinson Disease), (Caloric Restriction Mimetics as Priming Agents of Mesenchymal Stem Cells Secretome to Enhance Regenerative Responses to Parkinson’s Disease)
Each of these approaches addresses Parkinson’s in a distinct and scientifically grounded manner: cell replacement, neuroprotection, inflammation modulation, and regenerative support.
Clinical Evidence: A Tapestry of Progress
Early Safety and Signs of Efficacy
Two small studies published in April 2025 in Nature marked the revival of cell‑replacement therapy for Parkinson’s: injections of dopamine neuron progenitors derived from stem cells were found safe in both studies, involving 12 and 7 patients. Though not designed to prove efficacy, both hinted at symptomatic benefit, igniting guarded optimism in the medical community. No tumors, bleeding, or uncontrolled growth were observed, and immune suppression side effects were manageable. Early Parkinson’s trials revive stem cells as a possible treatment
Autologous iPSC Transplants in the U.S.
The trial led by Ole Isacson at McLean Hospital (affiliated with Harvard/Brigham) enrolled six Parkinson’s patients into a Phase 1 open‑label study using their own blood cells reprogrammed into dopamine neurons. (Clinical Trial Tests Novel Stem-Cell Treatment for Parkinson’s Disease)
Intranasal Neural Stem Cells in China
The Phase 1 trial using intranasal delivery of human neural stem cells (ANGE‑S003) enrolled 18 participants. It delivered dose‑escalating cells to the nasal mucosa, crossing into the brain. (Chinese studies on stem cells for Parkinson’s disease and sepsis)
MSC Trials Across the Globe
Mesenchymal stem cells are being tested globally, with safety and modest symptomatic benefits noted in early cohorts. For Parkinson’s, Phase 1 studies of umbilical‑cord MSCs intravenously have shown good tolerance; multiple trials (Mexico, China, Jordan, USA) continue to recruit or report early results. (Stem Cell-Based Therapies for Parkinson’s Disease), (Stem cell therapy for Parkinson’s disease: A new hope for neural regeneration)
How Stem Cell Therapy Can Help
Mesenchymal Stem Cells (MSCs) are adult stem cells known for their ability to reduce inflammation, repair damaged tissues, and modulate the immune system. These cells have the unique capability to home in on areas of damage or inflammation, making them ideal candidates for addressing complex neurological conditions like Parkinson’s. Their regenerative nature doesn’t just target symptoms—it helps support long-term cellular health. In the case of Parkinson’s, MSCs may offer support in the following ways:
- Neuroprotection: MSCs release growth factors that protect neurons from further damage.
- Reduction of Inflammation: They help calm the brain’s inflammatory response, a known contributor to Parkinson’s progression.
- Cell Communication: MSCs improve the cellular environment in the brain, potentially supporting better function of surviving neurons.
- Support for Dopamine-Producing Cells: While MSCs don’t directly replace lost neurons, they may improve the survival of existing dopamine-producing cells.
Why Choose Mesenchymal Stem Cell (MSC) Therapy Early
Early to mid-stage Parkinson’s patients may benefit most from MSC therapy. This is when there is still a significant number of functioning neurons that could be protected or supported. Starting therapy during this window maximizes the potential for regenerative healing and may help slow disease progression more effectively. It is also ideal for those who have reached a plateau in traditional treatments or are experiencing side effects from long-term medication use. Additionally, patients who wish to reduce their reliance on medications or delay the need for more invasive interventions may find MSC therapy to be a valuable option in their care plan.
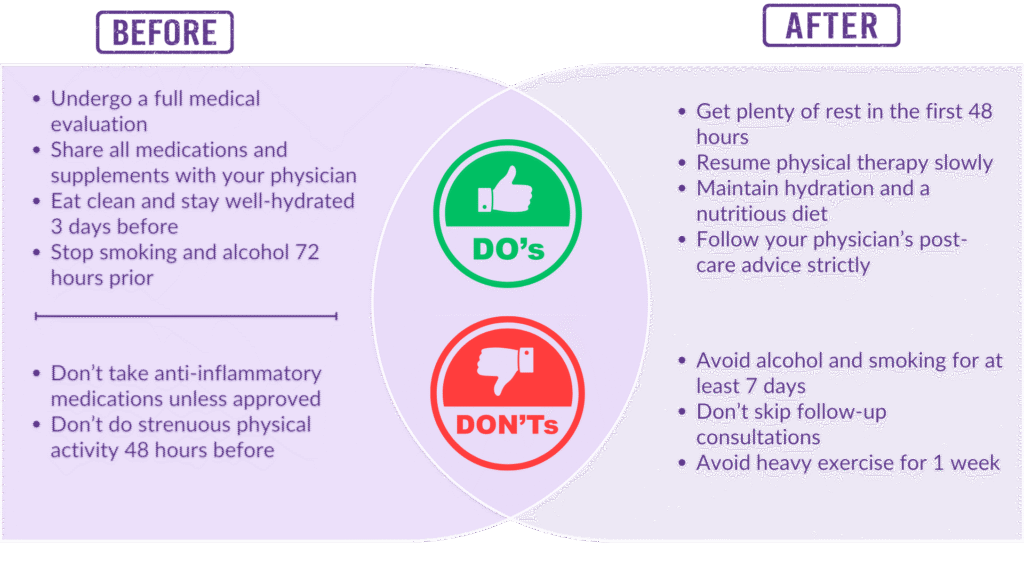
Before and After Stem Cell Therapy: What You Should Know
FAQs – Parkinson’s Disease & Stem Cell Therapy
At Doulton Healthwave, we go beyond traditional care by treating every patient with dignity, empathy, and a deep commitment to their well-being. We use only GMP-certified, ethically sourced umbilical cord-derived MSCs, selected for their purity, safety, and regenerative strength. Each cell is handled with the highest standards, ensuring every treatment is as safe and effective as possible. But beyond the science, we never forget the human side of healing. Our compassionate, patient-centered approach ensures your treatment plan is not just clinically sound — it’s also tailored to your unique condition, lifestyle, and hopes for recovery. Because here, you’re not just receiving care — you’re being cared for.